How Cold is Dry Ice? Unveiling Its Chilling Secrets
Dry ice is extremely cold, with a temperature of -78.5 degrees Celsius (-109.3 degrees Fahrenheit). It is a solid form of carbon dioxide and is commonly used for various purposes, such as freezing food, creating smoke effects, and preserving perishable items during shipping.
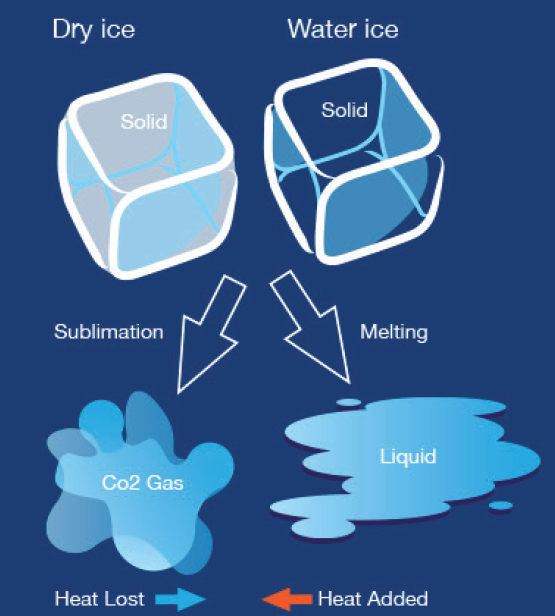
Dry ice is known for its frigid temperature, which is significantly colder than the freezing point of water. This extreme coldness is due to the fact that dry ice undergoes sublimation, transforming directly from a solid to a gas without becoming a liquid in between.
As a result, it can rapidly freeze objects upon contact, making it a valuable tool in various industries. In addition to its industrial applications, dry ice is also used for recreational purposes, such as creating spooky smoke effects in Halloween parties or adding a touch of drama to stage performances. However, it is important to handle dry ice with caution, as direct contact can cause frostbite.
The Nature Of Dry Ice
Dry ice is extremely cold, with temperatures plummeting to minus 109. 3 degrees Fahrenheit. It sublimates, turning from a solid to a gas without melting into a liquid, making it a unique and versatile cooling agent.
What Is Dry Ice?
Dry ice is solid carbon dioxide (CO2) that is extremely cold and has a temperature of -109.3°F (-78.5°C). It is called “dry” because it does not melt like regular ice, but instead, it sublimates, which means it turns directly from a solid to a gas.
Physical Properties
Dry ice is a white, odorless, and tasteless substance that is commonly used for cooling and freezing. It is denser than regular ice and has a specific gravity of 1.5, which means it sinks in water. Due to its extreme cold temperature, dry ice can cause frostbite if it comes into contact with skin, so it should always be handled with gloves or tongs. Dry ice is a unique substance that has many interesting properties. It is commonly used in the food industry for transportation and storage of frozen goods, as well as in the medical industry for preserving biological samples. It is also used in the entertainment industry for creating special effects, such as fog and smoke. Due to its extremely cold temperature and sublimation properties, dry ice requires careful handling and should never be ingested or used in confined spaces without proper ventilation. In conclusion, understanding the nature of dry ice is crucial in ensuring its safe and effective use. Its unique properties make it a valuable tool in various industries, but it should always be handled with care and caution.

Credit: www.quora.com
Temperature Details
Dry ice is extremely cold, with a temperature of -109. 3°F (-78. 5°C). This makes it ideal for keeping items frozen during transportation. Its sublimation process also creates a dramatic fog effect, perfect for special effects and scientific experiments.
Dry Ice’s Freezing Point
Dry ice freezes at -78.5°C (-109.3°F).
Comparison With Regular Ice
Regular ice freezes at 0°C (32°F).
Production Process
Dry ice is incredibly cold, reaching temperatures as low as -78. 5 degrees Celsius (-109. 3 degrees Fahrenheit). This extreme coldness makes it a popular choice for various production processes that require rapid freezing or preservation.
Creation Of Dry Ice
The production process of dry ice involves creating solid carbon dioxide (CO2) through a series of steps. It starts with capturing and compressing carbon dioxide gas from various sources such as industrial processes or natural gas wells. The captured CO2 is then purified to remove impurities and moisture. Once purified, the gas is pressurized and cooled to a low temperature using specialized equipment. The cold and pressurized CO2 gas is then released into a chamber where it expands rapidly, causing the gas to cool even further. This rapid expansion and cooling process results in the formation of solid carbon dioxide, commonly known as dry ice.
Commercial Manufacturing
Commercial manufacturing of dry ice involves large-scale production to meet the demand for various applications. The process starts with the same steps as the creation of dry ice, but on a much larger scale. The captured and purified carbon dioxide gas is compressed and cooled in industrial-sized chambers. These chambers are designed to handle high volumes of gas and produce large quantities of dry ice. The solid carbon dioxide is then collected, cut into desired shapes, and packaged for distribution. During the commercial manufacturing process, different forms and sizes of dry ice can be produced based on specific requirements. This includes blocks, pellets, and even customized shapes. The final dry ice products are carefully packaged to maintain their low temperature and prevent sublimation, which is the process of solid carbon dioxide turning directly into gas without melting into a liquid state. Overall, the production process of dry ice involves capturing, purifying, compressing, cooling, and shaping carbon dioxide gas to create solid carbon dioxide. This process ensures that dry ice is readily available for various industries and applications, including food storage, transportation, and special effects.
Safety Measures
Dry ice is extremely cold, with a temperature of -109. 3 degrees Fahrenheit. When handling dry ice, safety measures should be followed to avoid skin burns and frostbite. Always use insulated gloves and handle the dry ice with care to prevent any injuries.
When it comes to handling dry ice, safety should always be a top priority. Not only can it cause skin damage upon contact, but it can also release dangerous carbon dioxide gas if not handled properly. To ensure you handle and store dry ice safely, here are some important precautions you should follow.
Handling Precautions
- Always wear gloves or use tongs when handling dry ice to avoid skin damage.
- Never store dry ice in airtight containers as it can cause a build-up of carbon dioxide gas.
- Avoid touching dry ice directly with your skin or inhaling the gas it releases.
- When transporting dry ice, ensure the container is well-ventilated.
- Do not use dry ice in confined spaces as it can displace oxygen and cause suffocation.
Storage Guidelines
To store dry ice safely, there are some guidelines you should follow:
| Storage | Temperature | Duration |
|---|---|---|
| In a cooler | -109.3°F (-78.5°C) | Up to 24 hours |
| In a freezer | -109.3°F (-78.5°C) | Long-term storage |
When storing dry ice, always make sure to keep it in a well-ventilated area to prevent a build-up of carbon dioxide gas. It’s also important to note that dry ice will sublimate (turn into gas) over time, so be sure to use it within the recommended duration. By following these handling precautions and storage guidelines, you can safely use dry ice for a variety of purposes, from preserving food to creating spooky Halloween effects.
Uses In Various Industries
Food Preservation
Dry ice is widely used in the food industry for preserving perishable items such as fruits, vegetables, and meats. Its extremely cold temperature helps maintain the freshness of these products during transportation and storage.
Medical Field Applications
In the medical field, dry ice finds applications in the preservation and transportation of biological samples, vaccines, and pharmaceuticals. Its low temperature and ability to create a dry ice slurry make it ideal for preserving medical supplies that require constant refrigeration.

Credit: www.cganet.com
Scientific Experiments
Dry ice is a fascinating substance that has captivated scientists and researchers for decades. Its unique properties make it ideal for a wide range of scientific experiments, from educational demonstrations to cutting-edge research applications. In this section, we will explore how dry ice is used in scientific experiments and the various ways it contributes to our understanding of the world around us.
Educational Demonstrations
Dry ice is commonly used in educational demonstrations to engage students and teach them about the principles of science in a fun and interactive way. Its extreme cold temperature of -78.5 degrees Celsius (-109.3 degrees Fahrenheit) makes it a perfect tool for demonstrating the effects of low temperatures on different materials.
One popular demonstration involves placing a small piece of dry ice in a container of water. As the dry ice sublimates, it produces a thick, white fog that cascades over the sides of the container, creating a mesmerizing visual effect. This phenomenon, known as sublimation, allows students to observe the transition of a solid directly into a gas without passing through the liquid phase.
Dry ice can also be used to conduct experiments that demonstrate the principles of pressure and gas expansion. By sealing a small piece of dry ice inside a balloon and placing it in warm water, students can observe the balloon gradually inflate as the carbon dioxide gas produced by the sublimating dry ice expands. This simple experiment illustrates the relationship between temperature, pressure, and gas volume.
Research Applications
In addition to its educational uses, dry ice plays a crucial role in various research applications. Its extremely low temperature and ability to rapidly freeze substances make it valuable in fields such as chemistry, biology, and medicine.
One research application of dry ice is cryopreservation, which involves freezing biological samples, such as cells, tissues, and organs, for long-term storage. The low temperature of dry ice prevents the growth of microorganisms and slows down biochemical reactions, preserving the samples in a state of suspended animation until they are ready to be thawed and studied further.
Another research application is the use of dry ice to create controlled environments for studying the behavior of gases. By placing dry ice in a chamber with a specific gas, scientists can simulate conditions that mimic the atmosphere of other planets or explore the effects of different gas compositions on chemical reactions.
Furthermore, dry ice is utilized in cold trap systems to capture and remove unwanted gases or vapors during scientific experiments. Its low temperature allows it to efficiently condense and solidify gases, trapping them for analysis or disposal.
In conclusion, dry ice is not only a captivating substance but also a valuable tool in scientific experiments. Whether used for educational demonstrations or advanced research applications, its unique properties contribute to our understanding of the natural world and enable us to make important discoveries across various scientific disciplines.
Effects On The Environment
Dry ice is extremely cold, with a temperature of -78. 5°C. When it comes into contact with the environment, it can cause rapid sublimation, releasing carbon dioxide into the air. This can have negative effects on the environment, particularly if the dry ice is not handled or disposed of properly.
Environmental Impact
Dry ice sublimates directly into carbon dioxide gas without producing liquid waste.
Sustainability Considerations
Dry ice is produced from recycled carbon dioxide, reducing carbon footprint.
Credit: www.gulfcryo.com
Personal Experiences
Personal experiences with dry ice can be both fascinating and memorable. Let’s explore some firsthand encounters with this incredibly cold substance.
Anecdotes Of Dry Ice Usage
Using dry ice for special effects in a high school science experiment was both exciting and educational.
Creative Projects
Creating a spooky Halloween cauldron that bubbled and smoked using dry ice captured the attention of neighborhood kids.
Frequently Asked Questions
What Is Dry Ice Made Of?
Dry ice is solid carbon dioxide, formed by pressurizing and cooling CO2 gas until it reaches a temperature of -78. 5°C.
How Cold Is Dry Ice?
Dry ice is extremely cold, at a temperature of -78. 5°C, making it suitable for various cooling and freezing applications.
Is Dry Ice Safe To Touch?
Direct contact with dry ice can cause frostbite, so it’s important to handle it with protective gloves and avoid prolonged exposure.
How Long Does Dry Ice Last?
The longevity of dry ice depends on various factors, but it typically sublimates at a rate of 5-10 pounds every 24 hours in a standard cooler.
Conclusion
Understanding the temperature of dry ice is crucial for safe handling. Whether it’s for preserving food or creating special effects, knowing the extreme coldness of dry ice is essential. By being aware of its properties, individuals can harness its power while taking necessary precautions.
Stay informed and stay safe!
